About this article
I originally published this article on the Reef2Reef forum. It’s still available there along with some lively discussion: Establishing a Healthy Microbiome in a New Aquarium Using Live Rock
This article describes an experiment documenting the effects of live rock in a newly established aquarium. While I’ve tried to tell the story as briefly as I can, it’s the story of a month-long study of replicated tanks, with analysis of both water chemistry and the microbial communities in these tanks. The reader should be forewarned: I wasn’t able to distill the whole study into a quick soundbite.
Overall, I found that high-quality live rock promotes the rapid establishment of an effective biological filter and a microbial community similar to those found in mature reef tanks. Dry rock, on its own, showed no evidence of developing such a community during the first month. Interestingly, the benefits and microbial communities of live rock varied widely depending on the source.
Live rock and dry rock in new aquariums
Of all subjects in aquarium husbandry, the topic of how to best establish the microbial community in a new aquarium is one of the most debated. There is general agreement about the need to establish an effective biological filter with nitrifying microbes that convert ammonia and nitrite into the less toxic nitrate. Many aquarists also consider a diverse microbial community to be beneficial for other reasons, including disease prevention and control of unwanted algae or cyanobacteria. However, there are competing schools of thought about the best way to establish these communities.
For decades many aquarists have advocated the use of “live rock” to introduce beneficial microbes in a new aquarium. This term describes rock containing living populations of microbes from a saltwater environment, and includes a wide variety of material, including quarried rock, manmade rock, or pieces of coral rubble.
In contrast, many aquarists prefer to use “dry rock”. This term describes a similar range of materials, except in this case without a living microbial community. Advocates of this approach aim to prevent introduction of unwanted “hitchhikers”, the animals or algae that can accompany live rock.
Another approach that has grown in popularity in the recent decade is the use of products containing cultured bacteria. I have conducted experiments on this subject too and will describe those in a future report, but here I will focus on the effects of live rock itself.
An experiment to directly test the effects of live rock on the aquarium microbiome
Previous studies and the collective experience of the hobbyist community have shown that live rock is an effective tool for establishing a biological filter with nitrifying activity (i.e., the ability to convert ammonia into nitrite then nitrate).
However, we actually know very little about the effects of live rock on the microbial communities in new aquariums. To measure these effects, I set up a controlled and replicated experiment comparing the microbiomes of new aquariums established using live or dry rock.
For this experiment, I set up 6 identical systems using 20-gallon aquariums. Each of these experimental tanks is fully independent with its own heater, skimmer, powerhead, and return pump. They’re built as “all in one” systems; water drains from the display, then down through the rock chamber, then up through the pump chamber and back to the display (Figure 1). The display chambers are lit with black box LEDs, and tanks are covered with glass lids to minimize cross contamination, with a small hole remaining open for gas exchange.
 Figure 1: Self-contained nano tanks built for these experiments. (a) Diagram showing water flow through the tanks.
Figure 1: Self-contained nano tanks built for these experiments. (a) Diagram showing water flow through the tanks.
(b) A single tank shown during initial tests. (c) Eight experimental tanks arrayed in pairs on shelving units.
I established biological filtration in each of these tanks using one of three different materials:
Dry rock – dry, washed and sterilized Pukani rock
Live rock a – imported maricultured manmade rock
Live rock b – imported natural rock (coral rubble)
 Figure 2: Images of the live rock used in this experiment. (a) was sourced from a retailer, who described it as manmade rock cultured in Fiji. (b) was sourced from a wholesaler who described it as nano branch rock from mariculture facilities in Tonga.
Figure 2: Images of the live rock used in this experiment. (a) was sourced from a retailer, who described it as manmade rock cultured in Fiji. (b) was sourced from a wholesaler who described it as nano branch rock from mariculture facilities in Tonga.
I set up two identical tanks with each type of rock, and arranged these randomly on each shelving unit. I filled each tank with sterile-filtered (0.2 µm) UV-sterilized artificial seawater (NeoMarine – Brightwell Aquatics) prepared at 35 ppt using RODI water. In addition to the rocks, I added 4 lbs of dry aragonite sand to each tank.
Throughout the process, I was careful to control the microbial community introduced into each tank. For this reason, I sterilized tanks, equipment, sand, and dry rocks (but of course not live rock!) using bleach or hydrogen peroxide. While setting up, maintaining, and sampling these tanks, I sterilized my hands with bleach to minimize cross-contamination.
To fuel the growth of nitrifying microbes, I added ammonium sulfate to each tank to achieve a final ammonia concentration of 1 ppm. I measured ammonia every 1-3 days, and dosed as needed to restore each tank to 1 ppm. For the next month I continued to measure water chemistry and sample for microbiome testing, leaving the lights off to avoid algal growth during this period.
I measured water chemistry (ammonia, nitrate, and nitrite levels) using Red Sea and Seachem kits respectively. To correct for the interference of nitrite with the nitrate assay, I first measured nitrite then subtracted it from the nitrate values. Rather than visually evaluating the results (which can be imprecise and subjective), I read the absorbance of these tests at the appropriate wavelengths in a visible-wavelengths spectrophotometer (Model 721, an educational-grade instrument), and calculated concentrations based on comparisons to standard curves prepared using chemical standards made using reagent grade chemicals and an analytical balance.
To measure the microbial community in each tank, I followed the same procedures as in previous reports (here and here). Briefly: I sampled water from each tank at the beginning of the experiment, and again after 1, 2, and 3 weeks. I extracted DNA from each sample, and prepared these samples for DNA sequencing, focusing on a genetic marker called 16S commonly used to study microbial communities. I sequenced about 10,000 reads from each sample, and compared these with public databases (GreenGenes) to identify, classify, and measure the relative abundance of all microbes in these samples.
Live rock produces more diverse microbial communities than dry rock
These experiments showed that the choice of live or dry rock has profound and lasting effects on the microbial community. Communities in live rock tanks were much more diverse than dry rock tanks.
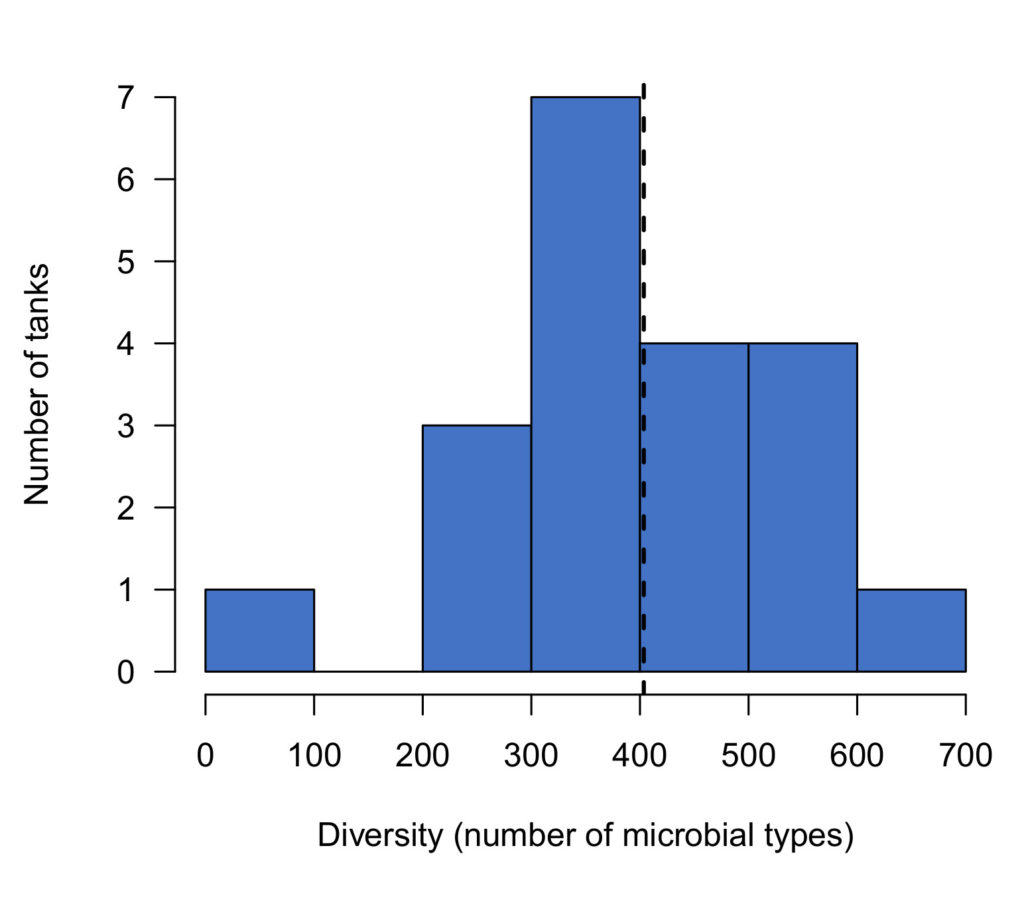
The diversity of a microbial community can be described simply in terms of the number of unique microbial types it contains. I found that unsurprisingly, tanks established using live rock contained much more diverse communities than those established with dry rock. This diversity advantage was already apparent shortly after establishing the tanks, and continued to increase throughout the experiment (Figure 3).
 Figure 3: Diversity of microbial communities in aquariums started with dry rock or live rock from different sources. Dashed lines indicate the 10th and 50th percentiles of diversity scores in a previous survey of established reef tanks.
Figure 3: Diversity of microbial communities in aquariums started with dry rock or live rock from different sources. Dashed lines indicate the 10th and 50th percentiles of diversity scores in a previous survey of established reef tanks.
Within the first 2 days after establishing these aquariums, live rock tanks already showed slightly greater diversity than dry rock (16-28% higher). Diversity in live rock tanks increased over the first two to three weeks, then remained relatively stable for the remainder of the experiment. The final diversity in live rock tanks was comparable to established reef tanks (average = 400 types). In fact, 20% of the established reef tanks we sampled recently (read more here) had lower diversity than the average in live rock tanks at the end of our experiment (325 types).
In contrast, the diversity in dry rock tanks was already slightly lower than live rock tanks, and it remained low throughout the experiment. At the end of the experiment, dry rock tanks were no more diverse than at the beginning: less than half that of live rock tanks, and only one third of that in established aquariums (Figure 3).
Live rock communities are like established aquariums, but dry rock communities are different
The composition and abundance of live rock communities was also more similar to established tanks than the communities in dry rock tanks. In a previous report, I described the ‘core aquarium microbiome’, a group of microbial families present in nearly all the established reef tanks I’ve sampled. Here, I compared the microbiome of live rock and dry rock tanks with this core microbiome to evaluate which approach was more effective in promoting microbial communities like those in established reef tanks.
In tanks started with live rock b, these core microbial families were generally present at levels similar to those in established aquariums. Dry rock tanks, in contrast, showed striking differences in the levels of these core microbial families. Dry rock tanks were deficient in some families, and enriched in other families when compared with established reef tanks.
 Figure 4: Examples of differences in the levels of core microbial families between tanks started with live rock or dry rock. Each panel shows the average abundance of each family. Typical levels are from a previous survey of 20 established reef tanks, and levels for dry or live rock represent the average of duplicate tanks.
Figure 4: Examples of differences in the levels of core microbial families between tanks started with live rock or dry rock. Each panel shows the average abundance of each family. Typical levels are from a previous survey of 20 established reef tanks, and levels for dry or live rock represent the average of duplicate tanks.
Examples of these differences are shown in Figure 4. One of two most abundant families in the core microbiome (Flavobacteriaceae) was also present at similarly high levels in live rock b communities, while dry rock communities were deficient in this family. Dry rock tanks showed similar deficiencies in Saprospiraceae and Cenarchaeaceae, while these families were present in live rock b tanks at levels similar to an established aquarium.
Other families were present in dry rock tanks at excessively high levels compared with established reef tanks. Hyphomicrobiaceae, Flammeovirgaceae, and Oceanospirillaceae are all found in established reef tanks, but these families were found at much higher levels in experimental tanks started with dry rock.
These family-by-family comparisons illustrate the differences between dry rock tanks and established reef tanks. To summarize these similarities and differences across all 19 of the core microbial families, I compared their relative levels in each sample with their average levels in the typical reef tank. I summarized these comparisons in a term I’m calling the “balance score”. (For readers familiar with statistics, this is simply the determination coefficient R2 calculated from a linear regression.) These scores range from 0 to 1, where 1 indicates a balanced community where the core microbial families are present at the same relative levels as in the average reef tank.
 Figure 5: Microbial communities in tanks started with live rock b are more similar to typical reef tanks than those in tanks started with dry rock or live rock a. Each bar shows the average score of duplicate identical tanks. Dashed lines indicate the 10th and 50th percentiles of balance scores observed a previous survey of established reef tanks.
Figure 5: Microbial communities in tanks started with live rock b are more similar to typical reef tanks than those in tanks started with dry rock or live rock a. Each bar shows the average score of duplicate identical tanks. Dashed lines indicate the 10th and 50th percentiles of balance scores observed a previous survey of established reef tanks.
Comparing these scores reveals that communities in live rock tanks were more balanced (i.e., more similar to mature reef tanks) than dry rock tanks. Balance scores in dry rock tanks were initially very low, and remained low throughout the experiment (Figure 5). In contrast, tanks started with live rock b showed high balance scores initially and throughout the experiment.
Comparing these scores with those obtained from established reef tanks provides important context for interpreting these differences. Live rock b communities had scores very similar to established reef tanks; in fact, the scores of these tanks exceeded those of nearly 50% of established reef tanks in our recent survey. In contrast, the scores of dry rock tanks were lower than >90% of reef tanks we sampled.
Together, these comparisons demonstrate that the microbial communities established using high-quality live rock are much more similar to an established reef tank than are communities established using dry rock.
Nutrient-processing microbes are much more abundant in aquariums started with live rock
Measuring the levels of specific microbes with known nitrifying activities revealed that these were present at much higher levels in experimental tanks started with high-quality live rock.
In the marine environment, ammonia is processed by ammonia-oxidizing Bacteria (AOB) including Nitrosococcus and several members of the Nitrosomonadaceae, and ammonia-oxidizing Archaea (AOA) in the family Cenarchaeaceae. AOA and AOB were present at low levels in all tanks throughout the experiment, but their abundance increased rapidly in live rock b (Figure 6a). By the end of the experiment, the total abundance of these groups was more than 14-times higher in tanks started with live rock b than in dry rock tanks. In fact, only 3 weeks after starting these tanks, the relative levels of AOB and AOB in these tanks were already higher than half of the established reef tanks we sampled. Dry rock tanks, in contrast, remained below the 10th percentile for these groups’ levels throughout the experiment.
 Figure 6: Relative levels of specific groups of microbes with nitrifying activity. Dashed lines indicate the median (50th percentile) level in a recent survey of established reef tanks. (a) Ammonia-oxidizing Bacteria and Archaea. (b) Nitrite-oxidizing Bacteria, NOB. Orange and green lines overlap in (b) because NOB were not detected in these water samples.
Figure 6: Relative levels of specific groups of microbes with nitrifying activity. Dashed lines indicate the median (50th percentile) level in a recent survey of established reef tanks. (a) Ammonia-oxidizing Bacteria and Archaea. (b) Nitrite-oxidizing Bacteria, NOB. Orange and green lines overlap in (b) because NOB were not detected in these water samples.
Nitrite is processed by nitrite oxidizing Bacteria (NOB) including Nitrobacter, Nitrococcus, and members of the families Nitrospinaceae, Nitrospiraceae, and Gallionellaceae. NOB were generally present at much lower levels overall than AOA and AOB; this can be best estimated at the end of the experiment in tanks started with live rock, where NOB were 18-times less abundant AOA+AOB. NOB were undetectable in all tanks at the beginning of the experiment, but these levels rose quickly in live rock b and became detectable within 3 weeks (Figure 6b). At that time, the relative levels of NOB in live rock b were already higher than half of the established reef tanks we sampled. In contrast, NOB remained undetectable in dry rock tanks throughout the experiment.
Interestingly, the growth of NOB in tanks started with live rock b perfectly mirrors the growth of the more abundant AOA & AOB (Figure 6a). This suggests that the populations of these groups develop simultaneously, so that levels of the more abundant AOA & AOB group may be sufficient for evaluating the maturity of a biofilter even if the less abundant NOB are not detected.
An important caveat for these findings is that these nutrient-processing microbes are primarily found in biofilms rather than free-living in the water column. The presence of these groups in aquarium water results from the recirculation of this water over biofilm-covered surfaces (e.g. pipes), and is evidence that they are present in the aquarium. However, their absence from some samples should not be interpreted as proof of absence in those tanks, since these groups are inherently less abundant in water samples than the biofilm. Instead, we can only say that microbes from these groups were below the limit of detection for this test (approximately 1 copy per 10,000, in this case), and were not detected.
Live rock is not all the same; the microbiome (and benefits) depend on the source
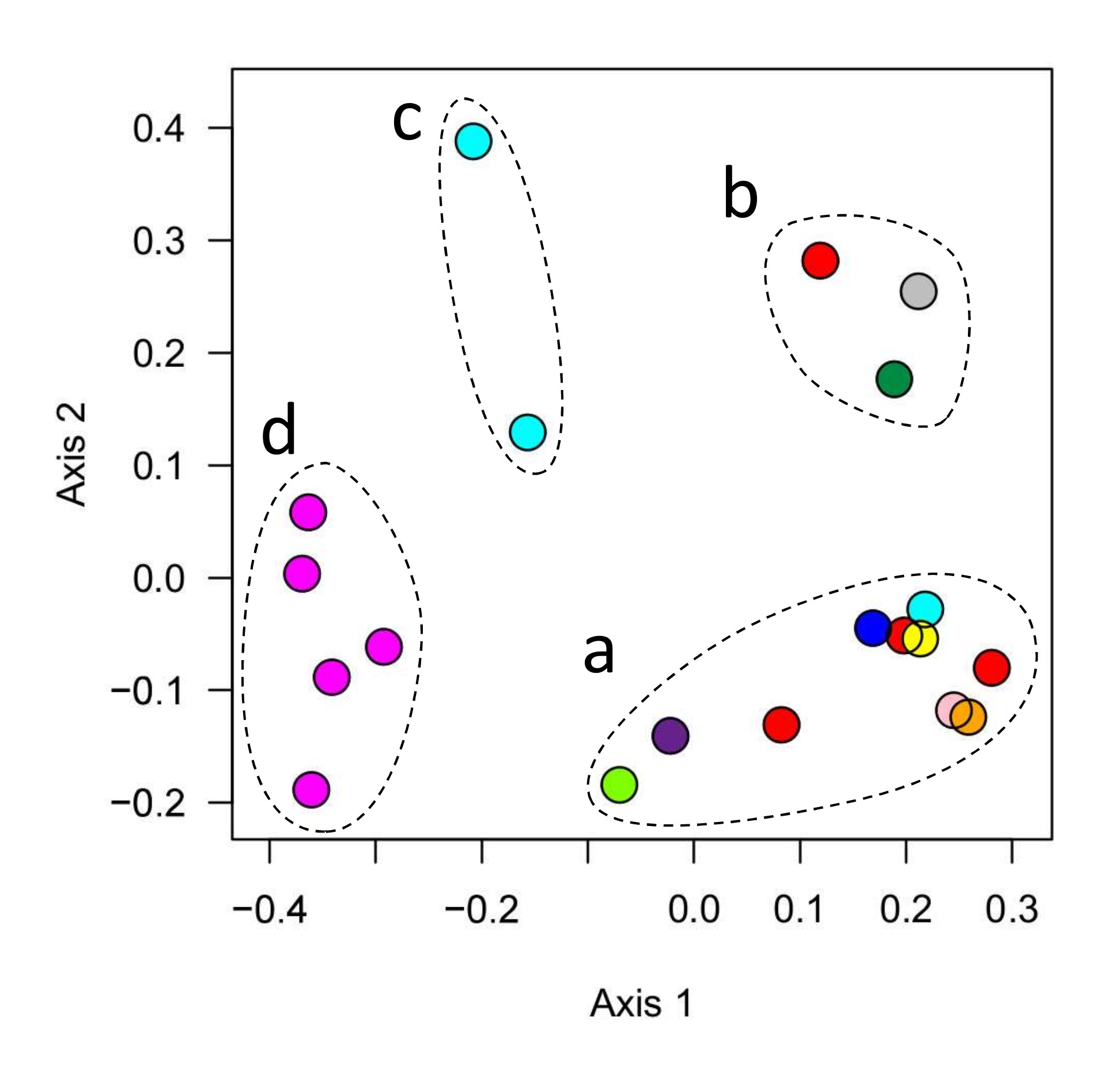
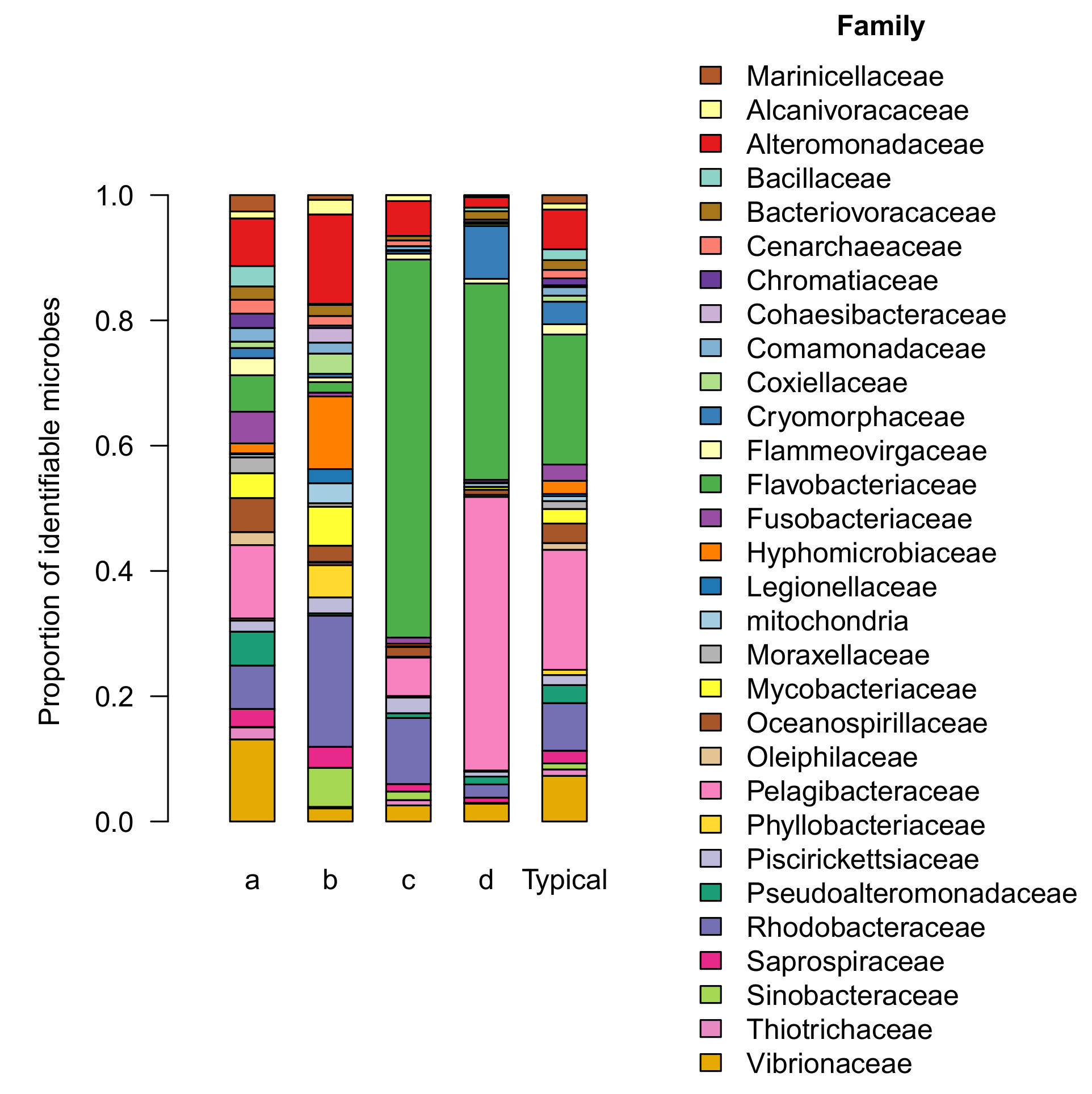
A careful reader may have noticed by now that the two sources of live rock used in this experiment produced very different communities. Although both sources promoted the growth of diverse microbial communities (comparable to the levels found in established reef tanks; Figure 3), the composition of these communities was very different.
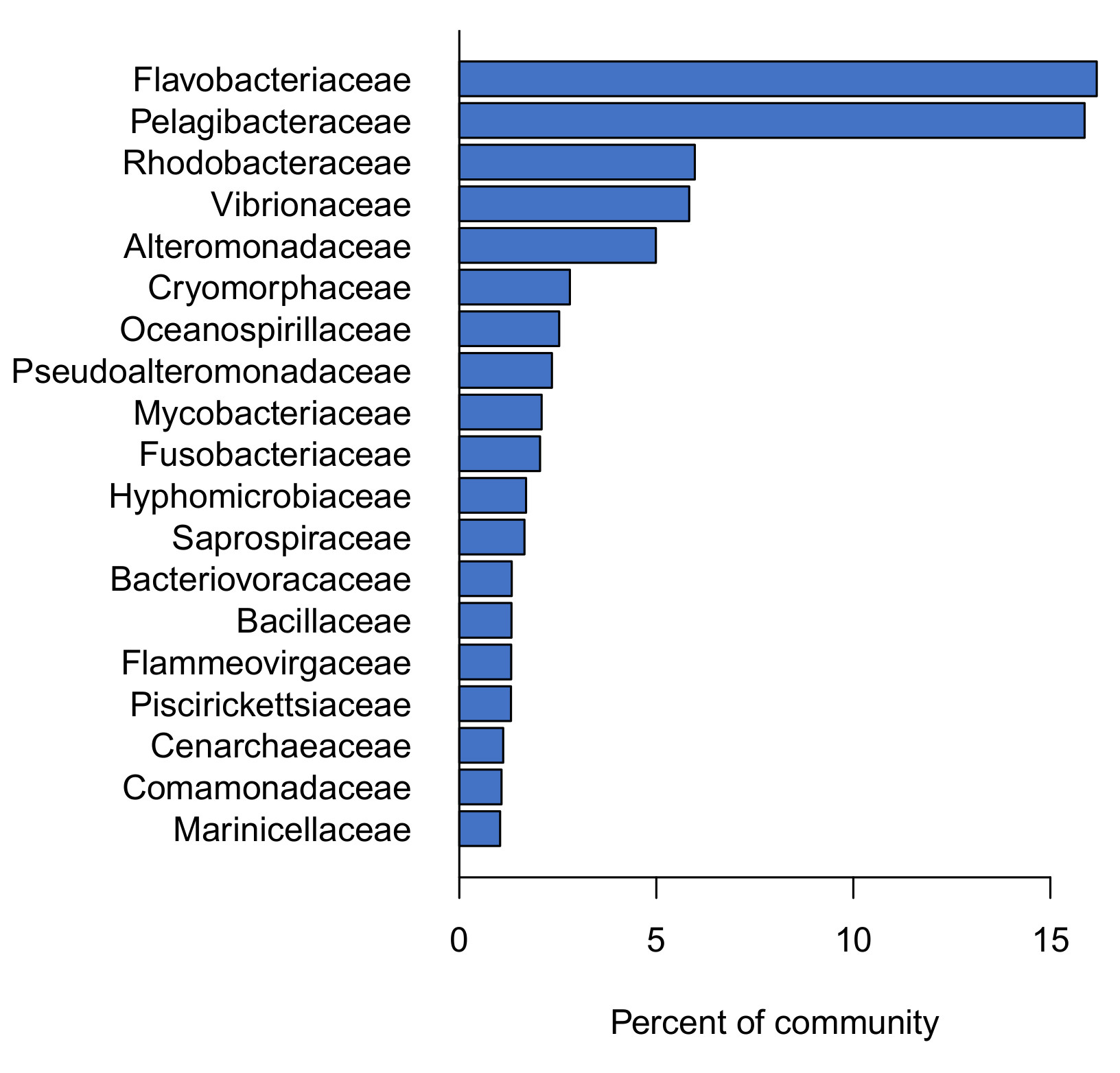
 Figure 7: Differences in the composition of microbial communities in tanks started with dry rock or live rock. All families making up at least 1% of the community in any group are shown. Each bar represents the average of duplicate tanks.
Figure 7: Differences in the composition of microbial communities in tanks started with dry rock or live rock. All families making up at least 1% of the community in any group are shown. Each bar represents the average of duplicate tanks.
These differences are clear when you compare the dominant families in each community (Figure 7). The community in experimental tanks started with live rock a was dominated by Flavobacteriaceae, which is also one of the top two most abundant families in the microbiome of a typical reef tank. The community in live rock b, in contrast, was dominated by Alcanivoraceae, which is not a major component of the typical reef tank’s microbiome (0.4%).
These communities were also different in their overall composition (Figure 7). The microbial families Criblamydiaceae, Parachlamydiaceae, and Kordiimonadaceae were abundant in live rock a but not in live rock b or in typical reef tanks. In contrast, the families Cenarchaeaceae and Flavobacteriaceae were abundant in both live rock b and typical reef tanks, but not in live rock a.
These differences in composition are also clear from the difference in balance scores (Figure 5). The low scores of live rock a indicate that the relative levels of the core microbial families were very different in these tanks than in typical reef tanks. In fact, these scores were comparable to dry rock tanks (Figure 5), despite the difference in composition and diversity of these communities (Figures 3, 7). This highlights the fact that while there is a single consensus microbiome shared by many reef tanks (read more details here), there are many different ways to deviate from this consensus. It also emphasizes that measurements of diversity alone are inadequate for describing the quality of an aquarium’s microbiome.
Overall this comparison between live rock sources a and b showed that these materials produced very different communities that were both more diverse than dry rock, but differed in their composition, balance, and levels of nitrifying microbes.
Live rock rapidly establishes an effective biological filter; dry rock does not
To evaluate the development of a biological filter in these tanks, I measured changes in ammonia, nitrite, and nitrate concentrations in all tanks throughout the experiment. These measurements clearly showed that live rock promoted the rapid development of nitrifying microbial communities, while dry rock tanks showed little or no evidence of nitrification activity throughout the experiment.
I maintained ammonia at approximately 1 ppm in each tank throughout the experiment to fuel the growth of these communities. To measure how quickly ammonia was consumed in each tank, I took a series of measurements after adding each dose of ammonia. Figure 8 show the ammonia consumption in 3- to 4-week old tanks, from the final week of the experiment. Microbes in live rock tanks consumed 100% of ammonia (reducing levels to zero) in less than 2 days, while dry rock tanks showed no change in ammonia during this period and in fact only consumed 7% of ammonia over the 6-day series of measurements (Figure 8).
 Figure 8. A series of measurements during the final week of the experiment, showing consumption of ammonia. Each symbol represents the average of duplicate tanks.
Figure 8. A series of measurements during the final week of the experiment, showing consumption of ammonia. Each symbol represents the average of duplicate tanks.
The growth of ammonia-oxidizing microbes can also be demonstrated by measuring the appearance of nitrite in aquarium water. Nitrite was detected within 18 days in live rock tanks (0.5 ppm), and levels continued to rise until the third week , then began to decline (Figure 9a). In contrast, nitrite remained undetectable in dry rock tanks throughout the experiment.

Figure 9. Production of nitrite and nitrate by microbial communities in each tank after while ammonia levels were maintained at approximately 1 ppm. Each symbol represents the average of duplicate tanks.
The growth of nitrite-oxidizing microbes can be indirectly measured from the appearance of nitrate in aquarium water. Nitrate was initially undetectable in all tanks, and first appeared in live rock tanks at 12- to 18-days (Figure 9b). Levels continued to rise in live rock tanks until the final week of the experiment. In contrast, nitrate remained undetectable in dry rock tanks throughout the entire month after establishing these tanks.
The differences between live and dry rock in these experiments were clear: live rock produced microbial communities that serve as an effective biological filter, and dry rock did not.
The differences in water chemistry between live rock sources were less obvious, but on closer examination it’s clear that live rock b established a biological filter more rapidly than live rock a. Tanks started with live rock b produced detectable nitrite (0.33 ppm) within 12 days after the first dose of ammonia, while nitrite levels remained negligible (0.06 ppm) in live rock a at this stage. After 6 more days (18 days total), tanks started with live rock a caught up and produced comparable nitrite levels (0.39 ppm).
The same patterns can be seen in accumulation of nitrate. Tanks started with live rock b produced detectable nitrate (3 ppm) within 9 days after the first dose of ammonia, while nitrate levels remained very low (0.4 ppm) in live rock a at this stage. It was 6 days later (day 18) before the tanks started with live rock a produced comparable nitrate levels (3.3 ppm).
Finally, the depletion of nitrite in live rock b by the end of the experiment, while nitrite levels remained fairly constant in live rock a (Figure 9a) is further evidence that live rock b promoted more rapid growth of nitrite-oxidizing microbes than live rock a.
[A reader familiar with the details of these tests may wonder if the similarity in these patterns results from interference of nitrite with the nitrite tests. In fact, to correct for this I subtracted the measured nitrite concentrations from nitrate data, removing at least the majority of this interference.]
Overall, these measurements of water chemistry demonstrate that live rock can establish an effective biological filter much more rapidly than dry rock, which failed to establish an effective biofilter at all within the one-month duration of this experiment.
These experiments also showed that the benefits of live rock differed depending on the source. High-quality live rock (b) developed both ammonia-oxidizing and nitrite-oxidizing activities more rapidly, achieving two of the traditional endpoints for cycling a tank (appearance of nitrate and depletion of nitrite) more quickly than low-quality live rock (a), which never depleted nitrite during the course of this experiment.
Finally, it is interesting to note that nitrifying microbes showed up in the microbiomes of live rock b tanks (Figure 5) at the same time as nitrite and nitrate appeared in their water (Figure 8). Meanwhile, these microbes were present at much lower levels in live rock a. This is consistent with the observation that the larger populations of nitrite-oxidizing microbes in live rock b were able to reduce nitrite levels to zero by the end of the experiment, while nitrite levels remained stable in live rock a.
This comparison also serves to illustrate a point made previously that undetected microbes are not necessarily absent. The chemistry data clearly show that live rock a contained some NOB, even though they were present at low levels below the limit of detection for DNA sequencing of water samples. Direct comparison of water and biofilm samples from a single tank confirmed that these microbes can be absent in the water column but present in biofilm. Based on these and related observations, we’re including both biofilm and water samples in all current and future testing, to increase our ability to detect these important microbial components of the biological filter.
High-quality live rock can also avoid unwanted algal blooms
This section is really a post-script to the original experiment. My original goal was to compare the microbial communities and nutrient processing activities of tanks set up with dry or live rock. After ending the cycling experiment I continued to maintain the tanks with minimal maintenance, adding a small amount of RODI water weekly to replace evaporation. I didn’t even clean the glass. In fact, the inner surface of the glass in these aquariums had never been cleaned in the 3 months since they were set up, at the time these photos were taken.
Two and a half months after starting the tanks, I turned on the lights to allow algal growth. Two weeks later (at 3 months) I added a small number of identical livestock to each tank (snails, hermit crabs, and a single fish – Halichoeres sp., probably melanurus).
At this stage, on observing the maturing tanks more closely, it became clear that in addition to the differences in nitrifying activities described above, the experiment also produced another effect. As the aquariums matured, different algal communities developed in each group, and the tanks started with high-quality live rock almost entirely avoided the ugly stages associated with unwanted algal blooms in new aquariums.

Figure 10. Photographs of the experimental tanks after approximately 3 months. All photos were taken with the same settings and lighting, and glass was never cleaned in any of these aquariums after the initial setup. Letters indicate IDs of the experimental tanks.
The differences are obvious and consistent within each group (Figure 10). The tanks started with either dry rock and live rock a show extensive algal growth on all surfaces, including sand, rocks, and glass. In contrast, tanks started with live rock b appeared dramatically cleaner, with no visible algal growth on sand or rocks and very little on the glass (See Figure 11 for a closer view of each example).

Figure 11. Closeup views of the sand and rock in a tank from each treatment group.
Because these strong effects were seen consistently across duplicate tanks, which were otherwise identical, we can be confident attributing these effects to the source of rock. Based on the striking differences in microbial communities already measured in these tanks during the first month of their development, I conclude that differences in microbial communities contributed to these effects. Further experiments will be needed to identify the specific microbial families responsible for these differences in algal communities, but the overall effects are clear.
Many aquarists have seen differences between the ugly stages in newly established aquariums, but few data from controlled experiments are available to determine the reasons for these differences. My findings demonstrate tanks started with live rock harboring microbial communities like those of established reef tanks (i.e., high balance scores) develop very little of the unwanted algal blooms often seen in new aquariums. This matches an observation made by many experienced reef keepers that tanks started with high-quality live rock can avoid the ugly stages. Together, these findings suggest that microbiome testing can be used to evaluate these communities before the ugly algal blooms ever develop.
High-quality live rock is recommended when starting a new aquarium
Based on these findings, from a microbiological perspective several conclusions are clear:
High-quality live rock promotes the rapid establishment of a microbial community like established reef tanks.
Dry rock does not develop such a community on its own in a similar time frame.
Live rock is not all the same, but differs in both microbial communities and biological filtration.
Live rock quality is not just a question of microbial diversity, but also community composition (not just how many types, but also which types).
The maturity of a biological filter, and the quality of live rock, can be measured through microbiome testing.
None of this addresses other reasons aquarists may favor dry rock or live rock (including aesthetics, ecological impacts, phosphate contents, or unwanted hitchhikers). But many aquarists use live rock specifically because of its microbial community, and this article provides some of the first data on these communities.
I found striking differences in the microbiome and biological filtration of different types of live rock. This naturally leads to the question, how can we identify high-quality live rock? Unfortunately, a study of only two sources doesn’t allow us to determine the reasons for differences between them. Live rock a and b differed in many ways including geographical origins (Fiji vs Tonga), porosity (solid vs porous), coverage with encrusting algae and invertebrates (high vs low), and composition (concrete and rubble vs natural coral skeletons).
All we can conclude with certainty is that appearances can be very deceiving. In this case, the prettier rock with extensive surface growth proved to be far inferior to the rock with very little surface growth. While it’s tempting to speculate that the highly porous structure of natural coral is important for high-quality live rock, further experiments are needed to determine whether that factor alone explains the difference. At present, it appears that if we want to know about the microbiome of live rock there is no substitute for measuring this directly, just like any other aspect of aquarium conditions (e.g. water chemistry or light intensities).
Speaking for myself, I won’t be starting any new tanks with dry rock unless I’m using them for an experiment. From a microbial perspective, it’s clear that live rock is an effective tool for rapidly establishing a microbial community like that of an established reef tank, and I’ve seen no evidence yet that dry rock – on its own – can achieve a similar community.
Since I know that will be a controversial statement I want to be precise about it. I am aware that many beautiful and successful reef tanks were started with dry rock – including some of those with the highest-scoring microbiomes in my previous survey of reef tanks.
What my controlled experiment shows is that diverse and balanced microbial communities do not just emerge automatically when dry rock is submerged in water. Instead, those microbes must be introduced. Successful reef keepers have come up with many ways of introducing beneficial microbes, including seeding the tank with small amounts of live rock rubble or live sand. Based on the experiments described here, I conclude that hobbyists who have succeeded in producing diverse and balanced microbial communities despite the use of dry rock have introduced beneficial microbes in some way… and I’ll be interested to hear how people have done so.
Further experiments are needed to systematically study the many products sold to promote a healthy microbiome in a new aquarium. By directly measuring the effects of these products using microbiome testing, the community can identify evidence-based best practices for starting a new aquarium with a healthy microbiome. We have a lot left to learn!
-Eli Meyer
AquaBiomics



 Figure 1: Self-contained nano tanks built for these experiments. (a) Diagram showing water flow through the tanks.
Figure 1: Self-contained nano tanks built for these experiments. (a) Diagram showing water flow through the tanks. Figure 2: Images of the live rock used in this experiment. (a) was sourced from a retailer, who described it as manmade rock cultured in Fiji. (b) was sourced from a wholesaler who described it as nano branch rock from mariculture facilities in Tonga.
Figure 2: Images of the live rock used in this experiment. (a) was sourced from a retailer, who described it as manmade rock cultured in Fiji. (b) was sourced from a wholesaler who described it as nano branch rock from mariculture facilities in Tonga. Figure 3: Diversity of microbial communities in aquariums started with dry rock or live rock from different sources. Dashed lines indicate the 10th and 50th percentiles of diversity scores in a previous survey of established reef tanks.
Figure 3: Diversity of microbial communities in aquariums started with dry rock or live rock from different sources. Dashed lines indicate the 10th and 50th percentiles of diversity scores in a previous survey of established reef tanks. Figure 4: Examples of differences in the levels of core microbial families between tanks started with live rock or dry rock. Each panel shows the average abundance of each family. Typical levels are from a previous survey of 20 established reef tanks, and levels for dry or live rock represent the average of duplicate tanks.
Figure 4: Examples of differences in the levels of core microbial families between tanks started with live rock or dry rock. Each panel shows the average abundance of each family. Typical levels are from a previous survey of 20 established reef tanks, and levels for dry or live rock represent the average of duplicate tanks. Figure 5: Microbial communities in tanks started with live rock b are more similar to typical reef tanks than those in tanks started with dry rock or live rock a. Each bar shows the average score of duplicate identical tanks. Dashed lines indicate the 10th and 50th percentiles of balance scores observed a previous survey of established reef tanks.
Figure 5: Microbial communities in tanks started with live rock b are more similar to typical reef tanks than those in tanks started with dry rock or live rock a. Each bar shows the average score of duplicate identical tanks. Dashed lines indicate the 10th and 50th percentiles of balance scores observed a previous survey of established reef tanks. Figure 6: Relative levels of specific groups of microbes with nitrifying activity. Dashed lines indicate the median (50th percentile) level in a recent survey of established reef tanks. (a) Ammonia-oxidizing Bacteria and Archaea. (b) Nitrite-oxidizing Bacteria, NOB. Orange and green lines overlap in (b) because NOB were not detected in these water samples.
Figure 6: Relative levels of specific groups of microbes with nitrifying activity. Dashed lines indicate the median (50th percentile) level in a recent survey of established reef tanks. (a) Ammonia-oxidizing Bacteria and Archaea. (b) Nitrite-oxidizing Bacteria, NOB. Orange and green lines overlap in (b) because NOB were not detected in these water samples. Figure 7: Differences in the composition of microbial communities in tanks started with dry rock or live rock. All families making up at least 1% of the community in any group are shown. Each bar represents the average of duplicate tanks.
Figure 7: Differences in the composition of microbial communities in tanks started with dry rock or live rock. All families making up at least 1% of the community in any group are shown. Each bar represents the average of duplicate tanks. Figure 8. A series of measurements during the final week of the experiment, showing consumption of ammonia. Each symbol represents the average of duplicate tanks.
Figure 8. A series of measurements during the final week of the experiment, showing consumption of ammonia. Each symbol represents the average of duplicate tanks.